|
SuperTeacherTools |
|

|

|
Scoring is built in for each team.
You can also choose to use a timer below.
|
Prefer the old Flash template? Switch now: Chem 213 Final Review SLG Winter 2024 Jeopardy Review Flash Version Chem 213 Final Review SLG Winter 2024 |
|
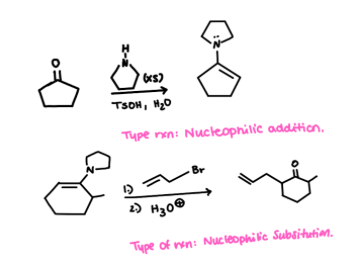
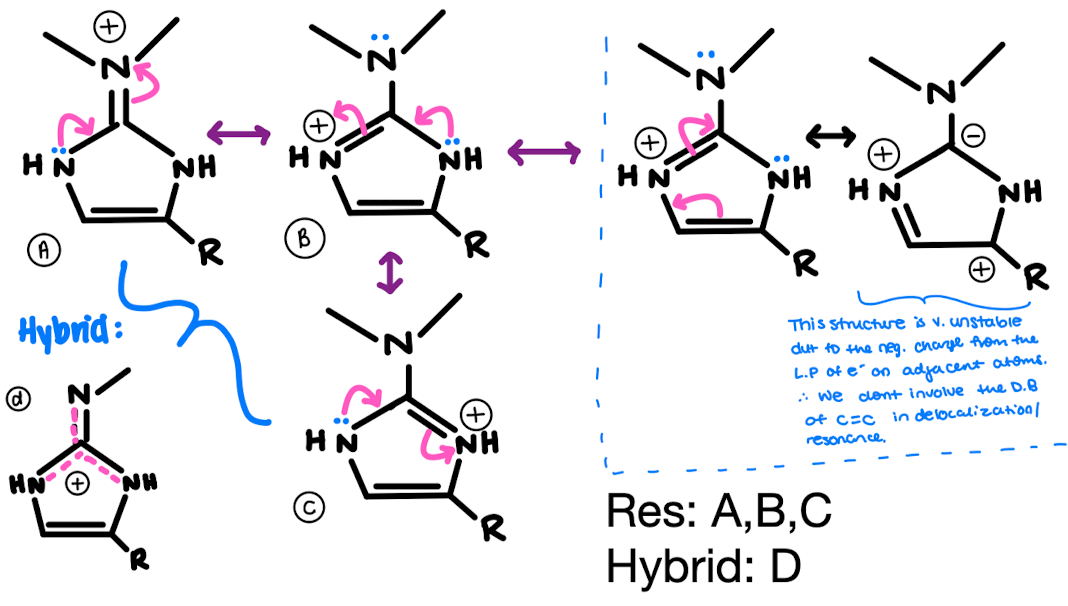
The dragmacidin class of natural products has been isolated from various marine sponges. They have been shown to have many interesting biological properties, including anti-viral, anti-fungal, and anti-bacterial activity. (+)-Dragmacidin D, shown below, was also made in the laboratory, which allowed the structure to be confirmed. The positive charge in dragmacidin D is delocalized. Draw all resonance structures that show delocalization of this charge, and then draw a resonance hybrid.
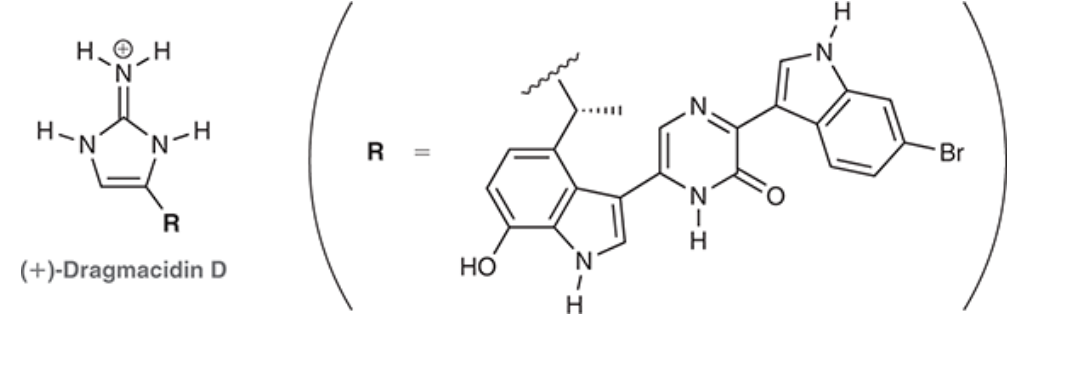

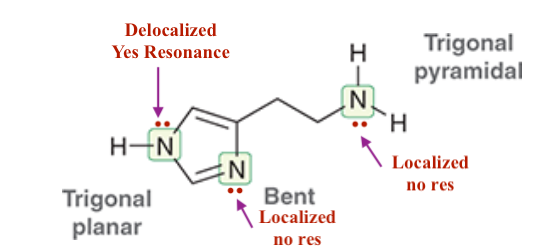
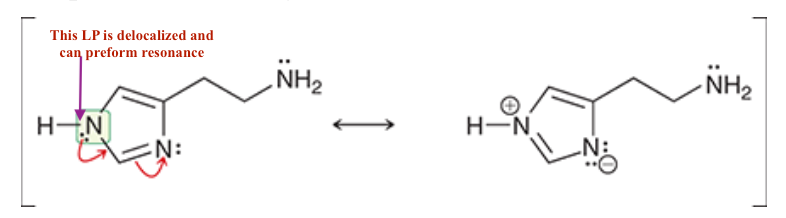
For Histamine:
1. ID where there are localized and de-localized lone pairs
2. What lone pairs can perform resonance -, show resonance.
3. Determine the hybridization of all N’s, and ID their geometry.
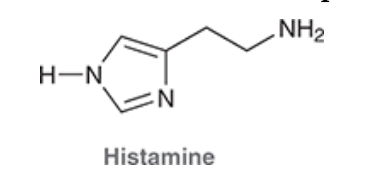
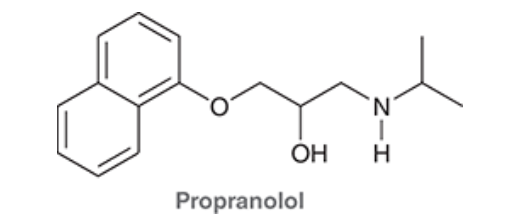
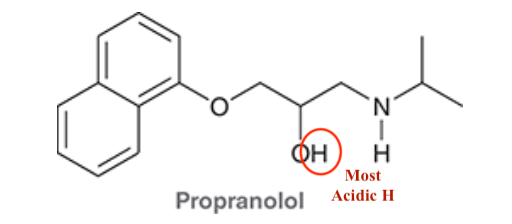
4. If Histamine N was to attack Propranolol what H would it take, why?

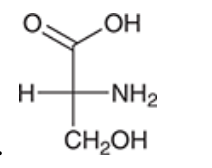
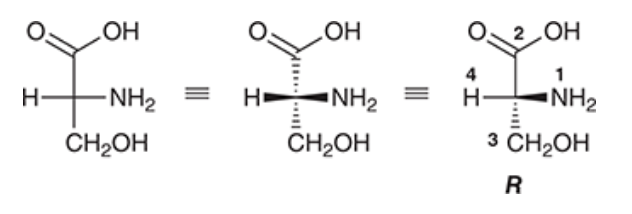
Identify the configuration of the chiral centre of the molecule, draw it in a wedge & dash.
Number and show configuration direction.

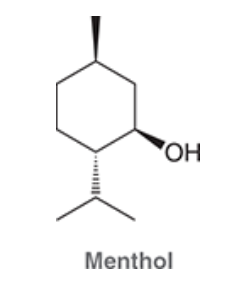
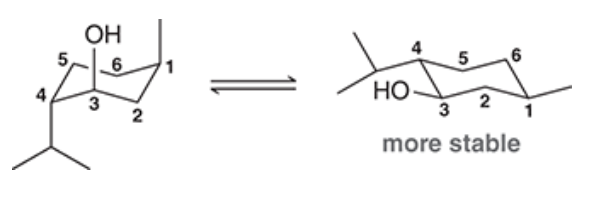
Menthol, isolated from various mint oils, is used in the treatment of minor throat irritation. Draw both chair conformations of menthol and indicate which conformation is lower in energy.

The following disubstituted cyclohexane, drawn in a Newman projection, was shown to have moderate antiviral activity

Consider the reaction below, which involves both a dehydrogenation (removal of two neighboring hydrogen atoms) and a Diels-Alder reaction (which we will learn about in Chapter 16). Utilizing a specially designed catalyst, the achiral starting materials are converted to a total of four stereoisomeric products—two major and two minor One of the major products is shown:
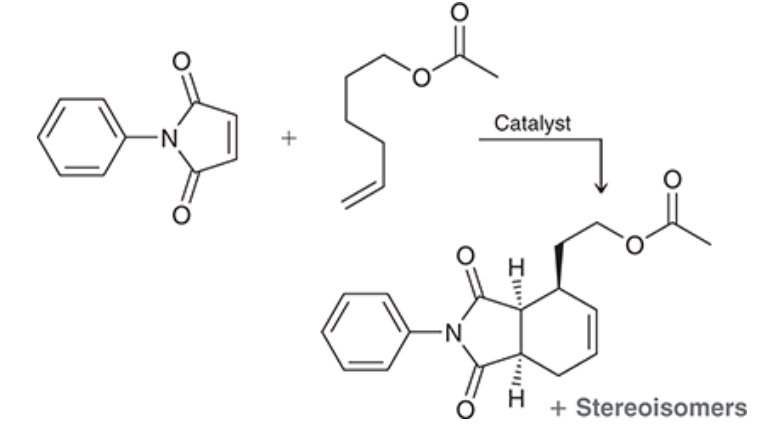
a) Draw the other major product, which is the enantiomer of the product shown.
b) The two minor products retain the cis connectivity at the bridgehead carbons but differ from the major products in terms of the relative stereochemistry of the third chiral center. Draw the two minor products.
c)What is the relationship between the two minor products?
d)What is the relationship between the major products and the minor products?

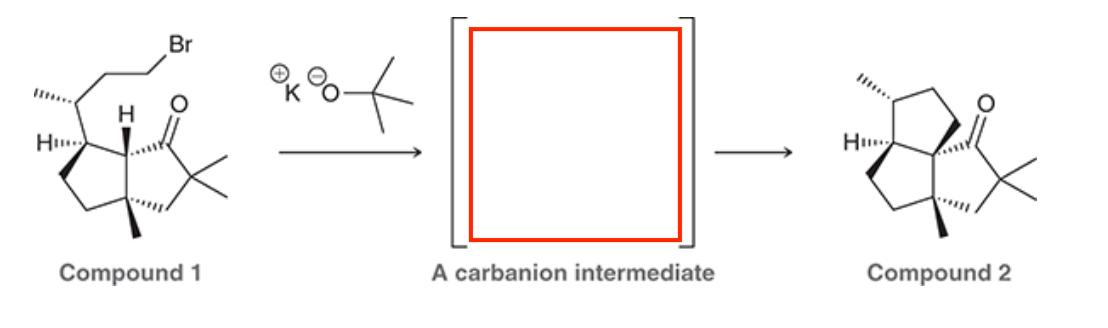
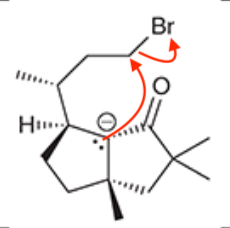
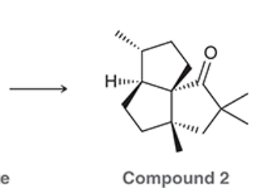
A common method for confirming the proposed structure and stereochemistry of a natural product is to synthesize the proposed structure and then compare its properties with those of the natural product. This technique was used to verify the structure of cameroonanol,1 a compound with a woody fragrance, isolated from the essential oil of the flowering plant Echniops giganteus. During the synthesis, compound 1 was deprotonated with a strong base to give a short-lived carbanion intermediate, which rapidly underwent an intramolecular SN2-type mechanism to afford compound 2.
A. Draw curved arrows to show the mechanism to get from the compound to the intermediate to the product.

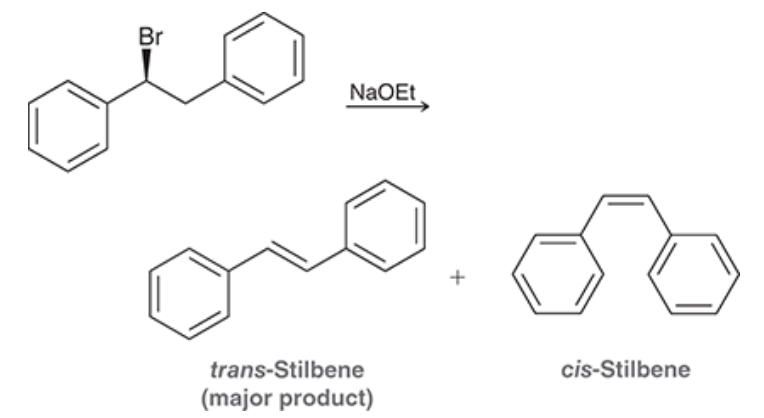
(S)-1-Bromo-1,2-diphenylethane (shown below) reacts with a strong base to produce trans-stilbene and cis-stilbene:

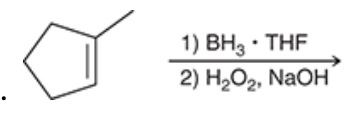
1. Complete the hydroboration-oxidation reaction.
2. hydroboration-oxidation produces what type of addition?

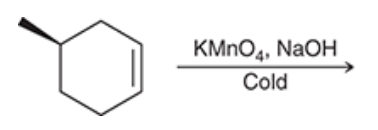
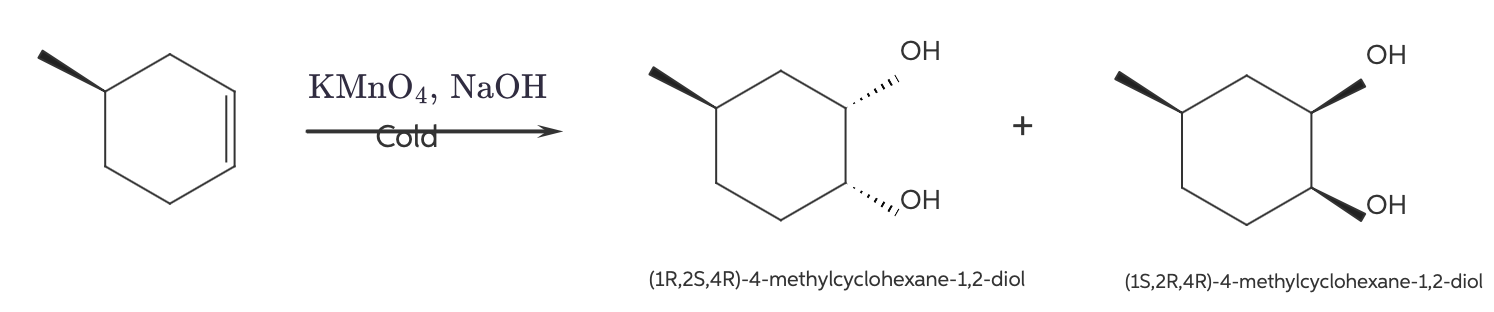
Syn dihydroxylation of the compound below yields two products. Draw both products and describe their stereoisomeric relationship (i.e., are they enantiomers or diastereomers?):

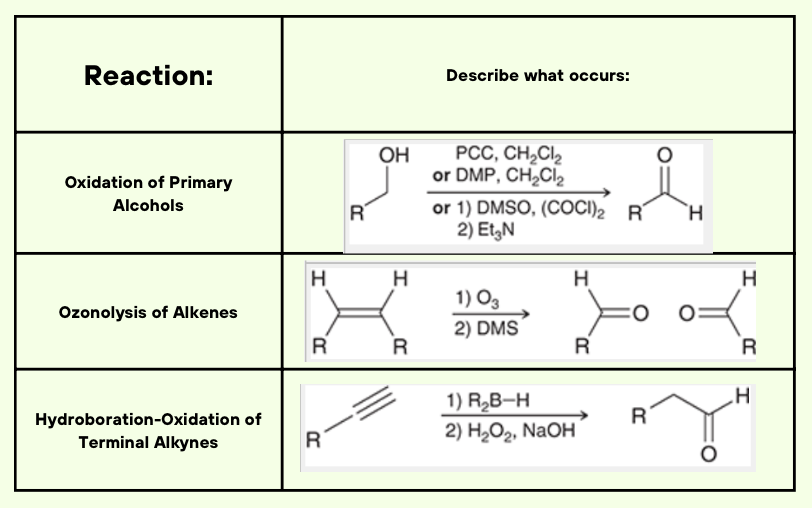
In words describe what is occuring within the reaction for either what we are treating it with, what will happen, or how it becomes the result:

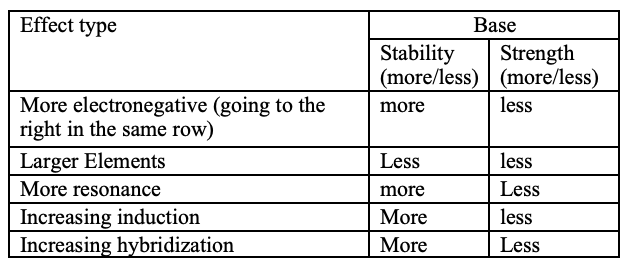
A= Atom
R= Resonance
I= Induction
O= Orbital
Ario is ranked in order of priority.
4.
O is more stable with the additional LP than N. Follows the Acid trend on PT.
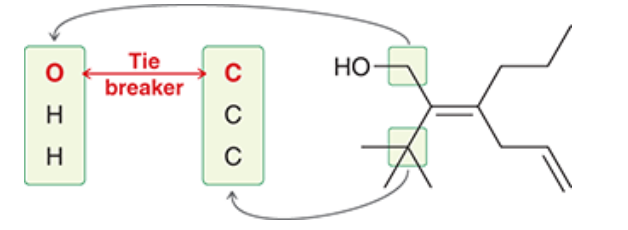
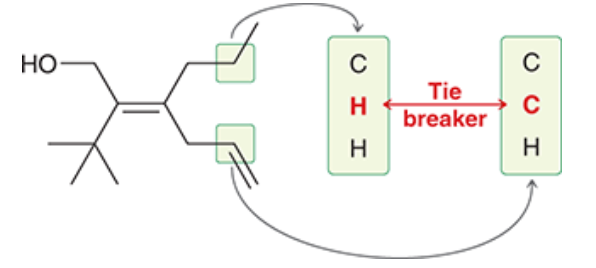
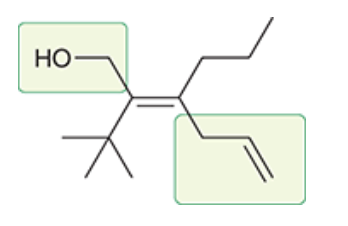
= E configuration
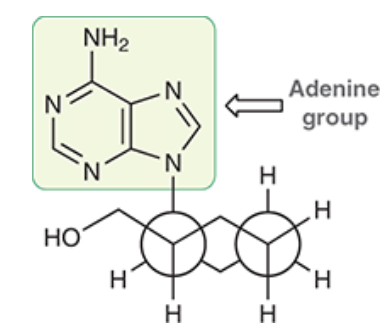
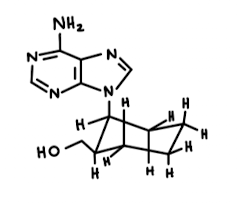
A. The adenine group is within the axial position. (but would be best suited in the equitorial). The CH2OH group occupying an equatorial position.
B.
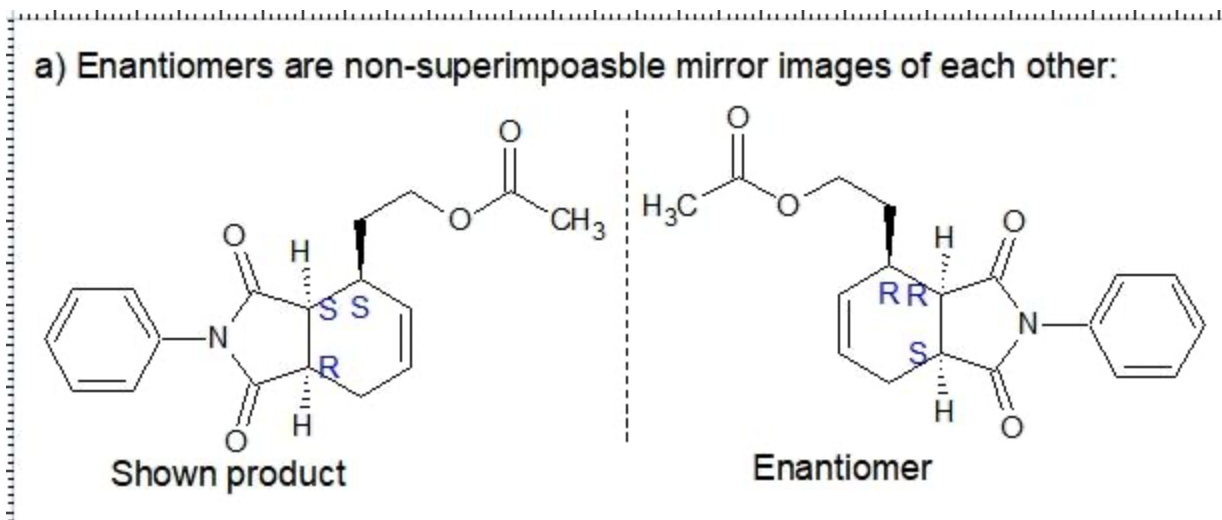
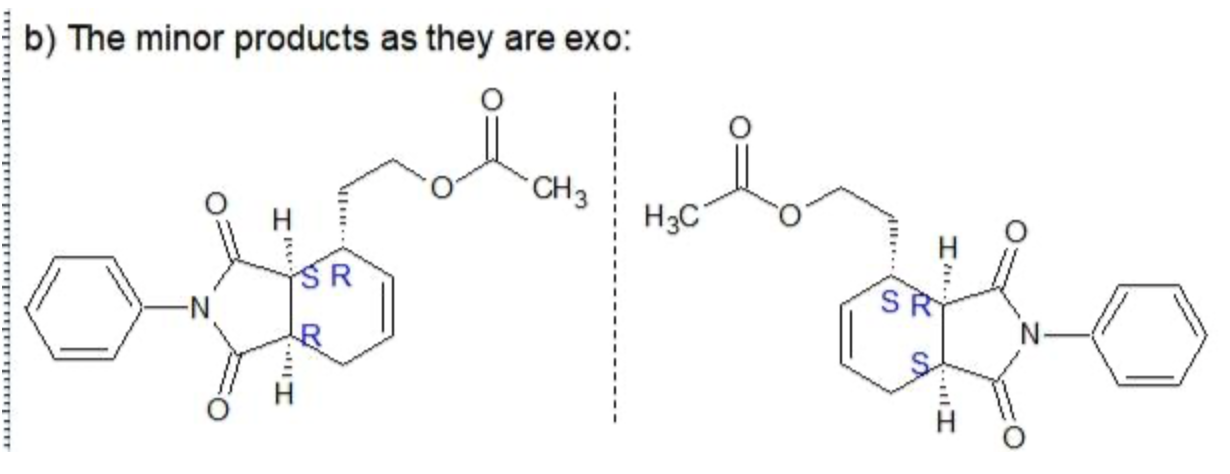
C. The minor products are non-superimposable mirror images so therefore they are Enatimomers
D. The major and minor products are not mirror images, so they are diastereomers
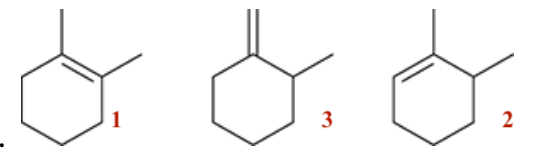
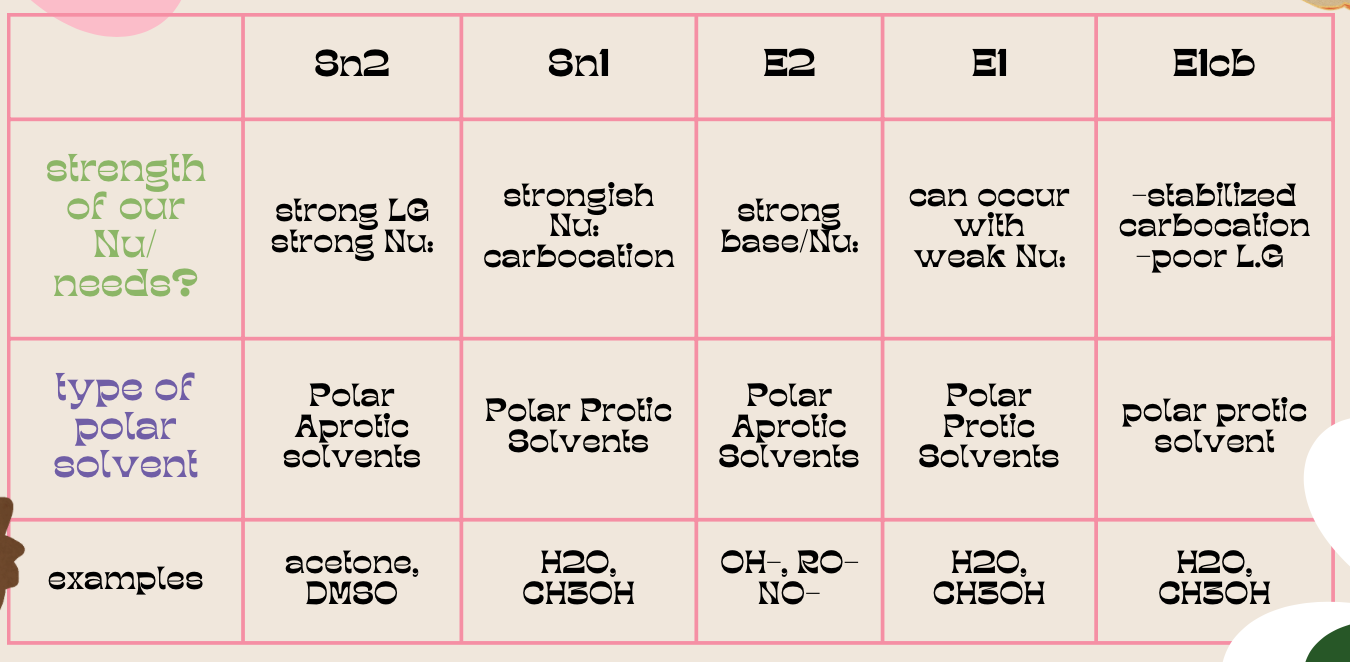
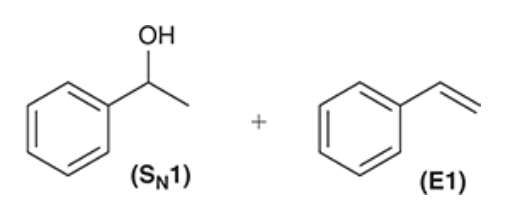
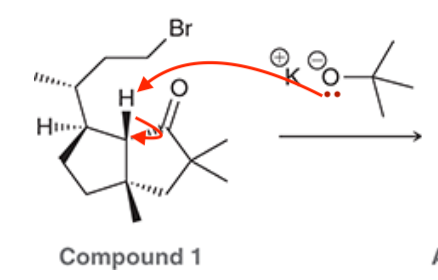
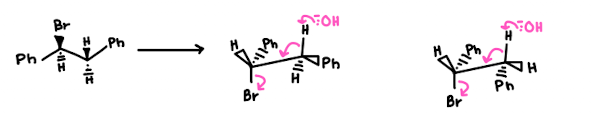
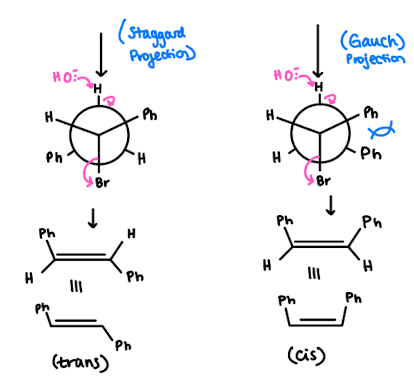
- we NaOEt which is a strong base and hence we are doing an E2 reaction
- There is less steric hindrance within the trans compared to the cis due to our close Ph groups - so Trans is more favourable
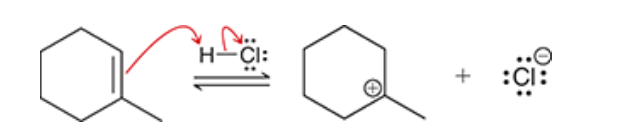
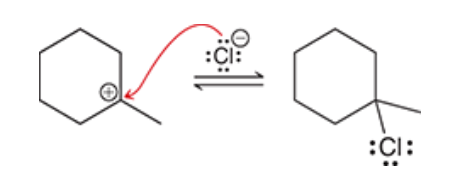
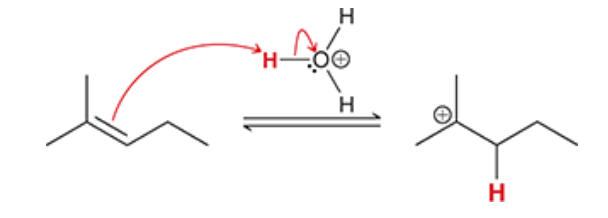
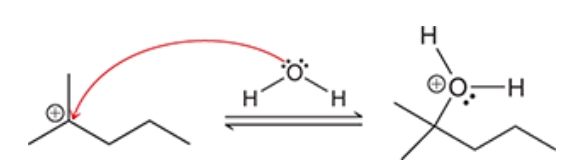
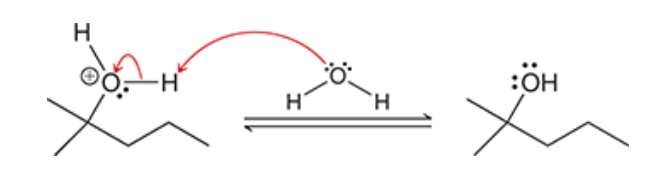
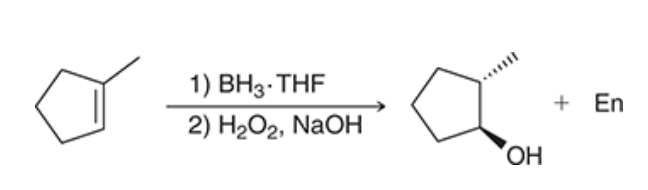
2. hydroboration-oxidation produces an anti-Markovnikov addition, which means that the OH group is installed at the less substituted position
The given reaction of syn dihydroxylation gives two products which are diastereomers
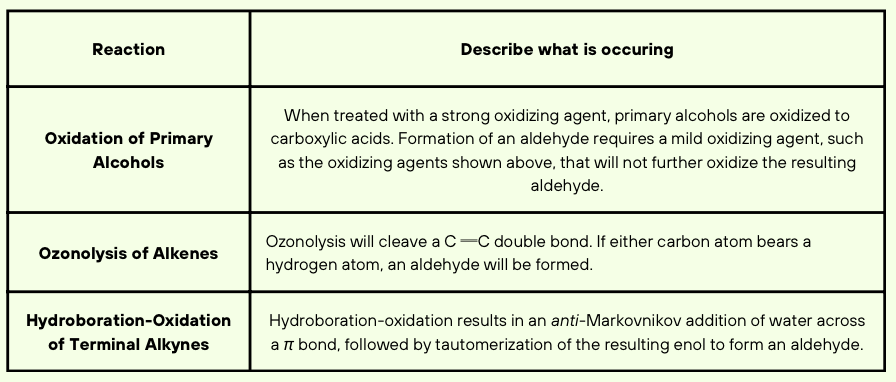
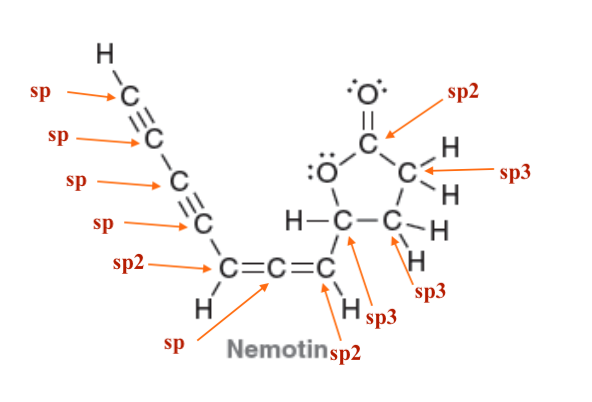
Each nitrogen atom has a lone pair that is adjacent to a ∏ bond and is, therefore, delocalized via resonance. In order to be delocalized via resonance, the lone pair must occupy a p orbital, and therefore, each nitrogen atom must be sp2 hybridized. As such, each nitrogen atom is trigonal planar.
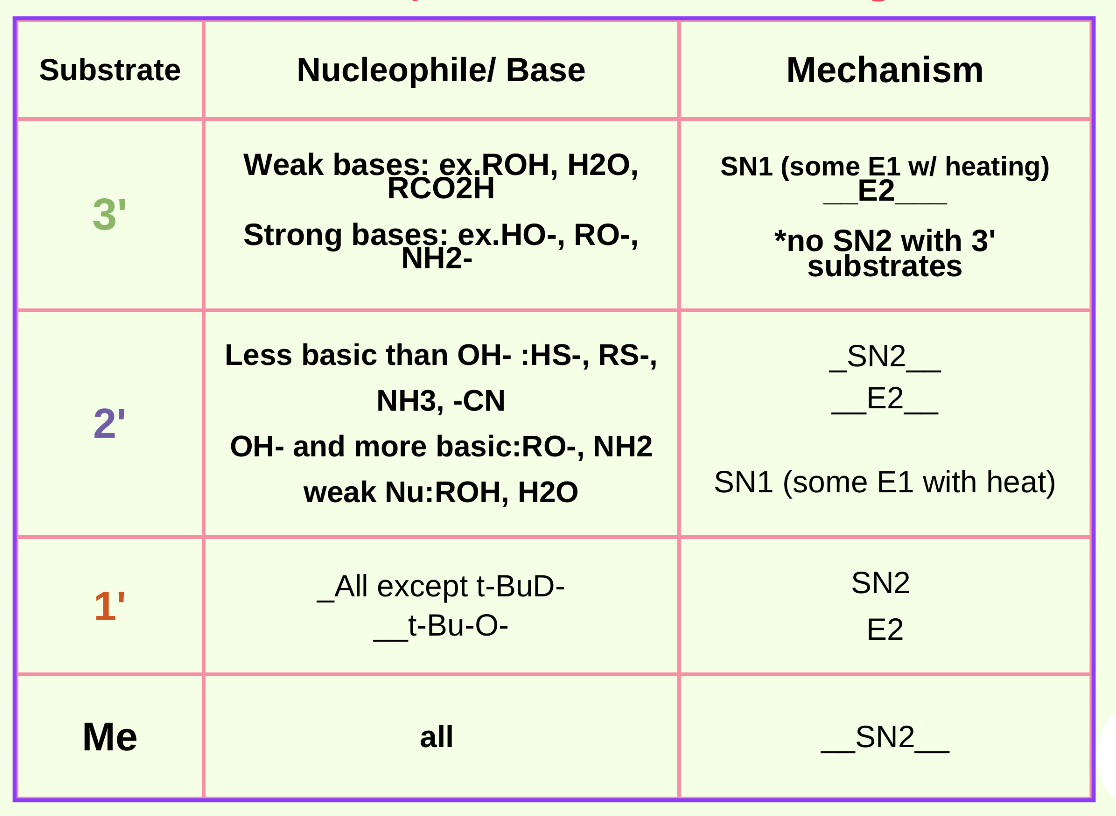
Answer:
ADEG or AIHG