How many electrons can be accommodated in all the atomic orbitals that correspond to the principal quantum number?

Which of the following has 1 electron located in a p orbital: C, F, H, N, or Al

Which of the following element is expected to have the highest melting point: Li, Ca, Rh, At, or C

Describe the ionization trend among sodium, magnesium, and aluminum

The intermolecular force that is common for all molecules is?

Which of the following would represent a nonpolar molecule that contains polar bonds: iodine, carbon dioxide, trifluorophosphine, or water?

Does ethanol have the capability to form a hydrogen bond?

Which end of the bonds in HCl, IBr, and CO would be more negative? (WAIT FOR ANSWER CHOICES)


How many grams of oxygen gas can be produced from the explosion of 454.2 grams of nitroglycerin?

The total pressure of a mixture of gasses is the sum of the individual pressures.

Volume is inversely proportional to pressure as temperature is held constant.

At constant volume, the pressure exerted by a given mass of gas varies directly with the absolute temperature.

Volume is proportional to the absolute temperature of a gass as pressure is held constant.

Three identical ballons are filled with equal volumes of three separate gases( water vapor, ozone, and xenon). All of the balloons are at 27 Centigrade and 1.5 atm pressure. Which balloon has the greatest kinetic energy?

The technique where a solution of a known concentration is used to determine the concentration of an unknown solution.

A substance that increases the rate of a chemical reaction without itself being consumed is known as a

An increase in temperature causes a/n ______________ in reaction rate

Calculate the molarity of an acetic acid solution if 34.57 mL of this solution are needed to neutralize 25.19 mL of 0.1025 M NaOH.

Explain how the orientation of particles affect the reaction rate.

The total quanitity of energy in the universe is assumed to remain constant is known as the...

100. grams of water is heated 10 degrees C. The specific heat of water is 4.184 J/gC. How much heat is absorbed by the water?

In the reaction NH3(g) + HCl(g) and#8594; NH4Cl(s), the entropy change is - or +?

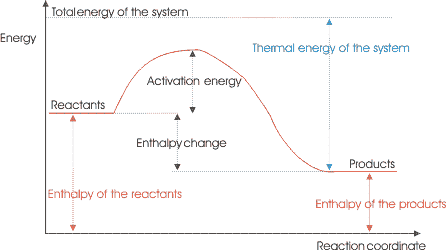
Exothermic or Endothermic?

Explain how entropy and enthalpy changes when ice melts.


32
Al
Sc3+
C
Ionization energy increases as nuclear charge increases and larger atoms require less energy to remove a valence electron.
Londer dispersion forces
trifluorophosphine
Yes. Draw a Lewis Structure and remember F, O, or N must be bonded to a hydrogen atom in the molecule.
A
16.00 g
Dalton's Gas Law
Boyle's Law
#!!##!!##!!#- Lussac's Law
Charles' Law
The kinetic energy is the same because the temperature for each balloon is equivalent.
Titration
catalyst
increase
7.469e-2 M
If particles do not successfully collide in the correct orientation, then the chemical change will not occur.
First Law of Thermodynamics
q= mcT= 4.18 kJ
Gases change to a more orderly solid state. The entropy change is negative.
Exothermic
Both entropy and enthalpy increases.















