|
Chemistry Review Jeopardy NNMS 2013
What are the three parts of a hypothesis?

What are the three parts of a hypothesis?
If... Then... Because...

What in class activity served as a model for the unknowns of science (e.g., deep space, atomic structure, origin of life on earth, etc.)?

What in class activity served as a model for the unknowns of science (e.g., deep space, atomic structure, origin of life on earth, etc.)?
THE BOX!

What is a simple representation that allows scientists to explain their observations and make predictions?

What is a simple representation that allows scientists to explain their observations and make predictions?
Model

What two branches of science fall under the umbrella of Physical Science?

What two branches of science fall under the umbrella of Physical Science?
Chemistry and Physics

Who said ESSENTIALLY ALL MODELS ARE WRONG, BUT SOME ARE USEFUL?

Who said ESSENTIALLY ALL MODELS ARE WRONG, BUT SOME ARE USEFUL?
George E. P. Box

What is the center of the atom called?

What is the center of the atom called?
Nucleus

What is the name of the negatively charged particle inside of an atom?

What is the name of the negatively charged particle inside of an atom?
Electron

Whose model of the atom is pictured below? 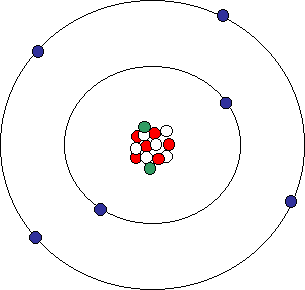

Whose model of the atom is pictured below? 
Bohr

Who had an atomic model that was like PLUM PUDDING or CHOCOLATE CHIP COOKIE DOUGH

Who had an atomic model that was like PLUM PUDDING or CHOCOLATE CHIP COOKIE DOUGH
J. J. Thomson

What was Murray Gell-Mann's contribution to atomic theory?

What was Murray Gell-Mann's contribution to atomic theory?
Quarks

Are periods vertical or horizontal?

Are periods vertical or horizontal?
Horizontal

What is the name of the vertical columns on the periodic table?

What is the name of the vertical columns on the periodic table?
Groups (or Families)

What gets added when you move down to the next period?

What gets added when you move down to the next period?
An Electron Shell

What is the name of the family with completely filled electron shells?

What is the name of the family with completely filled electron shells?
Noble Gases

What makes chlorine a violent element?

What makes chlorine a violent element?
It needs an electron to have a full shell, so it will take the electron from anywhere. (It'll take an electron from kittens)

What type of mixture is the same throughout?

What type of mixture is the same throughout?
Homogeneous

What is one sign of a chemical change?

What is one sign of a chemical change?
New substance, color change, gas formed, temperature change, light given off

Name a type of physical change.

Name a type of physical change.
Breaking, bending, mixing, phase change

What is name of the phase change for when a solid turns to a gas?

What is name of the phase change for when a solid turns to a gas?
Sublimation

What is the name of the theory that describes the motion of elements in the different states of matter?

What is the name of the theory that describes the motion of elements in the different states of matter?
Kinetic Molecular Theory

Which physical property does not depend on the amount of substance? Intensive or extensive?

Which physical property does not depend on the amount of substance? Intensive or extensive?
Intensive

What is the formula for density?

What is the formula for density?
D = m/v

What is the name for the temperature when a substance goes from a liquid to a gas?

What is the name for the temperature when a substance goes from a liquid to a gas?
Boiling point

What is the density of a substance with a mass of 5 grams and a volume of 2 ml?

What is the density of a substance with a mass of 5 grams and a volume of 2 ml?
2.5 ml

What is the mass of 6 ml of barium if the density of barium is 3 g/ml?

What is the mass of 6 ml of barium if the density of barium is 3 g/ml?
18 g

What is chemistry?

What is chemistry?
Chemistry is the study of matter, energy, and the interactions between them.


| Team 1 |
 |
|
 |
|
| Team 2 |
 |
|
 |
|
| Team 3 |
 |
|
 |
|
| Team 4 |
 |
|
 |
|
| Team 5 |
 |
|
 |
|
| Team 6 |
 |
|
 |
|
| Team 7 |
 |
|
 |
|
| Team 8 |
 |
|
 |
|
| Team 9 |
 |
|
 |
|
| Team 10 |
 |
|
 |
|

What Would You Like To Risk?

| Team 1 |
 |
|
 |
|
| Team 2 |
 |
|
 |
|
| Team 3 |
 |
|
 |
|
| Team 4 |
 |
|
 |
|
| Team 5 |
 |
|
 |
|
| Team 6 |
 |
|
 |
|
| Team 7 |
 |
|
 |
|
| Team 8 |
 |
|
 |
|
| Team 9 |
 |
|
 |
|
| Team 10 |
 |
|
 |
|
Go To The Final Question
Final Score:

| Team 1 |
 |
|
 |
|
| Team 2 |
 |
|
 |
|
| Team 3 |
 |
|
 |
|
| Team 4 |
 |
|
 |
|
| Team 5 |
 |
|
 |
|
| Team 6 |
 |
|
 |
|
| Team 7 |
 |
|
 |
|
| Team 8 |
 |
|
 |
|
| Team 9 |
 |
|
 |
|
| Team 10 |
 |
|
 |
|

Edit This Game:

|
|











