|
ubaTaeCJ
I discovered that atoms are mostly ____________.

I discovered that atoms are mostly ____________.
Empty space


In Rutherford's experiment, a thin gold foil was bombarded with alpha particles, what was his hypothesis?

In Rutherford's experiment, a thin gold foil was bombarded with alpha particles, what was his hypothesis?
Alpha particles should have passed through the foil with little or no deflection.

My model differed from Thompson’s model because the protons were in the ______, not spread out with the electrons.

My model differed from Thompson’s model because the protons were in the ______, not spread out with the electrons.
Center


In 1897, he demonstrated that ______ ____, which were unknown at the time, were made of unknown negative charges.

In 1897, he demonstrated that ______ ____, which were unknown at the time, were made of unknown negative charges.
Cathode Rays


J. J. Thomson’s experiments provided evidence that atoms are made up of even smaller particles. He found particles within the atom that have a ________ charge.

J. J. Thomson’s experiments provided evidence that atoms are made up of even smaller particles. He found particles within the atom that have a ________ charge.

Thomson is usually credited for finding the first evidence of _____. (He did this while testing with canal rays, commonly referred to as or positive ions.)

Thomson is usually credited for finding the first evidence of _____. (He did this while testing with canal rays, commonly referred to as or positive ions.)
Isotopes

Rutherford discovered that the positive charges of an atom were located in a tiny, more massive area in the center of the atom in what year?


Rutherford discovered that the positive charges of an atom were located in a tiny, more massive area in the center of the atom in what year?

1911


In what year did Ernest Rutherford conduct an experiment to study the parts of the atom? (His experiment suggested that atoms have a nucleus—a small, dense center that has a positive charge and is surrounded by moving electrons. Rutherford later found that the nucleus is made up of smaller particles. He called the positively charged particles in the nucleus protons.)

In what year did Ernest Rutherford conduct an experiment to study the parts of the atom? (His experiment suggested that atoms have a nucleus—a small, dense center that has a positive charge and is surrounded by moving electrons. Rutherford later found that the nucleus is made up of smaller particles. He called the positively charged particles in the nucleus protons.)
1909

Thomson discovered the electron and created the plum pudding model in this year.


Thomson discovered the electron and created the plum pudding model in this year.


I am a negatively charged particle.

I am a negatively charged particle.
Electron
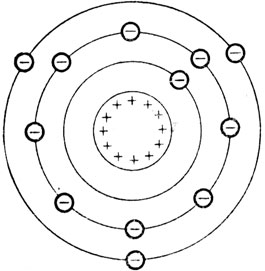

The electrrons are located here.

The electrrons are located here.
Electron Cloud


I am a positively  charged particle. charged particle.

These two particles make up the nucleus.

These two particles make up the nucleus.
Protons and Neutrons


These particles have no charge.

These particles have no charge.
Neutrons



| Team 1 |
 |
|
 |
|
| Team 2 |
 |
|
 |
|
| Team 3 |
 |
|
 |
|
| Team 4 |
 |
|
 |
|
| Team 5 |
 |
|
 |
|
| Team 6 |
 |
|
 |
|
| Team 7 |
 |
|
 |
|
| Team 8 |
 |
|
 |
|
| Team 9 |
 |
|
 |
|
| Team 10 |
 |
|
 |
|

What Would You Like To Risk?

| Team 1 |
 |
|
 |
|
| Team 2 |
 |
|
 |
|
| Team 3 |
 |
|
 |
|
| Team 4 |
 |
|
 |
|
| Team 5 |
 |
|
 |
|
| Team 6 |
 |
|
 |
|
| Team 7 |
 |
|
 |
|
| Team 8 |
 |
|
 |
|
| Team 9 |
 |
|
 |
|
| Team 10 |
 |
|
 |
|
Go To The Final Question
Final Score:

| Team 1 |
 |
|
 |
|
| Team 2 |
 |
|
 |
|
| Team 3 |
 |
|
 |
|
| Team 4 |
 |
|
 |
|
| Team 5 |
 |
|
 |
|
| Team 6 |
 |
|
 |
|
| Team 7 |
 |
|
 |
|
| Team 8 |
 |
|
 |
|
| Team 9 |
 |
|
 |
|
| Team 10 |
 |
|
 |
|

Edit This Game:

|
|


