
|
SuperTeacherTools |
|

|

|
Sb2F5
NEGATIVE
-ΔG = SPONTANEITY
Trigonal Planar
OXIDIZING AGENT
CHEMICAL CHANGE
NITROGEN
NET IONIC EQUATION
IRON (III) PHOSPHATE
PROTON, NEUTRON, ELECTRON
K= 273.15 + 32°C
305 K
2+
2
METALLOIDS
EXOTHERMIC
THE NEUTRAL ATOM'S LIKLIHOOD OF GAINNING AN ELECTRON.
P4O6
Se2-
IONIC
Z = ATOMIC NUMBER
CHLORINE GAS (CL2) IS BEING REDUCED.
SILICON TETRACHLORIDE
Would you use an oxidizing agent or reducing agent in order for the following reactions to occur?
Mn2+ ![]() MnO2
MnO2
WHAT IS THE OXIDATION NUMBER OF Mg IN THE COMPOUND MgO?
WHAT'S THE SYMBOL FOR AN ION THAT CONTAINS 34 PROTONS AND 36 ELECTRONS?
TRUE OR FALSE?
AT EQUILIBRIUM THE CONCENTRATIONS OF REACTANTS AND PRODUCTS NO LONGER CHANGE.
WHAT IS THE TOTAL NUMBER OF VALENCE ELECTRONS IN AN ATOM OF Ba?
TRUE or FALSE?
TEMPERATURE HAS NO EFFECT ON EQUILIBRIUM.
IS THE FOLLOWING COMPOUND IONIC OR COVALENT ?
NH4OH
WHAT IS THE NAME OF THE FORMULA?

WHAT IS THE SIGN (+/-) ON ΔG FOR A SPONTANEOUS PROCESS?
WHAT IS THE DEFINITION OF ELECTRON AFFINITY?
HOW MANY ELECTRONS MUST BE ADDED, AND TO WHAT SIDE DO YOU ADD ELECTRONS TO BALANCE AND COMPLETE THE HALF-REACTION?
WHAT DOES THE Z REPRESENT IN THE ISOTOPE NOTATION?

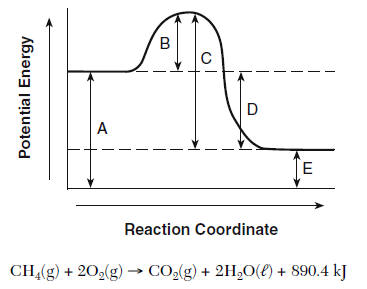
WHICH LETTER REPRESENTS THE ACTIVIATION ENERGY OF THE REACTION?
What is 32°C in Kelvin?
(3 Sig Figs)
WHAT'S THE FORMULA OF ANTIMONY PENTAFLOURIDE?
WHAT IS THE FORMULA OF Tetraphosphorus Hexoxide ?
PHYSICAL or CHEMICAL CHANGE?
DECOMPOSING WATER BY PASSING AN ELECTRIC CURRENT THROUGH IT.
WHAT ARE THE NAMES OF THE THREE SUBATOMIC PARTICLES ?
WHAT'S THE NAME OF THE FORMULA?

COMPLETE THE SENTENCE:
REMOVING THE SPECTATOR IONS OF AN EQUATION LEAVES YOU WITH
THE ?
IN THE FOLLOWING HALF-REACTION, WHICH REACTANT IS BEING REDUCED?
Mg(s) + Cl2 (g) → MgCl2 (s)


| Description | Match: |
WHAT ARE THE ELEMENTS IN THE CIRCLED AREA CALLED?
|
METALLOIDS |
WHICH ATOM HAS THE LARGER IONIZATION ENERGY?
Mg (Magnesium) or N (Nitrogen)
|
NITROGEN |
WHAT'S THE SYMBOL FOR AN ION THAT CONTAINS 34 PROTONS AND 36 ELECTRONS? |
Se2- |
WHAT IS THE TOTAL NUMBER OF VALENCE ELECTRONS IN AN ATOM OF Ba? |
2 |
WHAT IS THE DEFINITION OF ELECTRON AFFINITY? |
THE NEUTRAL ATOM'S LIKLIHOOD OF GAINNING AN ELECTRON. |
COMPLETE THE SENTENCE:
REMOVING THE SPECTATOR IONS OF AN EQUATION LEAVES YOU WITH THE ?
|
NET IONIC EQUATION |
WHAT IS THE OXIDATION NUMBER OF Mg IN THE COMPOUND MgO?
|
2+ |
HOW MANY ELECTRONS MUST BE ADDED, AND TO WHAT SIDE DO YOU ADD ELECTRONS TO BALANCE AND COMPLETE THE HALF-REACTION?
Cl2 → 2 Cl- | ADD 2 ELECTRONS TO THE REACTANT (RIGHT) SIDE
Cl2 + 2 e- → 2 Cl- |
IN THE FOLLOWING HALF-REACTION, WHICH REACTANT IS BEING REDUCED?
Mg(s) + Cl2 (g) → MgCl2 (s) |
CHLORINE GAS (CL2) IS BEING REDUCED. |
Would you use an oxidizing agent or reducing agent in order for the following reactions to occur?
Mn2+ |
OXIDIZING AGENT |
WHAT IS THE NAME OF THE FORMULA?
|
SILICON TETRACHLORIDE |
WHAT IS THE FORMULA OF Tetraphosphorus Hexoxide ? |
P4O6 |
WHAT'S THE FORMULA OF ANTIMONY PENTAFLOURIDE? |
Sb2F5 |
WHAT'S THE NAME OF THE FORMULA?
|
IRON (III) PHOSPHATE |
IS THE FOLLOWING COMPOUND IONIC OR COVALENT ?
NH4OH |
IONIC |
TRUE or FALSE?
TEMPERATURE HAS NO EFFECT ON EQUILIBRIUM. | |
EXOTHERMIC OR ENDOTHERMIC REACTION?
|
EXOTHERMIC |
WHAT IS THE SIGN (+/-) ON ΔG FOR A SPONTANEOUS PROCESS? |
NEGATIVE
-ΔG = SPONTANEITY |
TRUE OR FALSE?
AT EQUILIBRIUM THE CONCENTRATIONS OF REACTANTS AND PRODUCTS NO LONGER CHANGE. | |
WHICH LETTER REPRESENTS THE ACTIVIATION ENERGY OF THE REACTION?
|
|
WHAT ARE THE NAMES OF THE THREE SUBATOMIC PARTICLES ? |
PROTON, NEUTRON, ELECTRON |
PREDICT THE THREE DIMENSIONAL SHAPE OF THE FOLLOWING MOLECULE.
|
Trigonal Planar |
What is 32°C in Kelvin?
(3 Sig Figs) |
K= 273.15 + 32°C
305 K |
PHYSICAL or CHEMICAL CHANGE?
DECOMPOSING WATER BY PASSING AN ELECTRIC CURRENT THROUGH IT. |
CHEMICAL CHANGE |
WHAT DOES THE Z REPRESENT IN THE ISOTOPE NOTATION?
|
Z = ATOMIC NUMBER |